Bacterial Microcompartments
What are Microcompartments?
Bacterial microcompartments, or MCPs (or alternatively BMCs) are large structures found within many bacteria. These fascinating supramolecular structures function as simple protein-based organelles. MCPs are roughly polyhedral in shape and are comprised of a thin protein shell that encapsulates various metabolic processes. The outer protein shell is assembled from thousands of tessellating protein subunits, in a fashion that is reminiscent of a viral capsid. The basic building block of this protein shell is the BMC domain which goes on to form the outer hexameric and trimeric shell proteins. The first studies MCP is the carboxysome. It encapsulates the enzymes carbonic anhydrase and RuBisCO in cyanobacteria and some chemoautotrophs in order to enhance CO2 fixation. Other types of microcompartments have been studied that metabolize two and three carbon compounds such as ethanolamine and 1,2-propanediol in various enteric bacteria. These microcompartments play a role in optimizing metabolic reactions and preventing the escape of toxic and volatile intermediates into the cytosol.
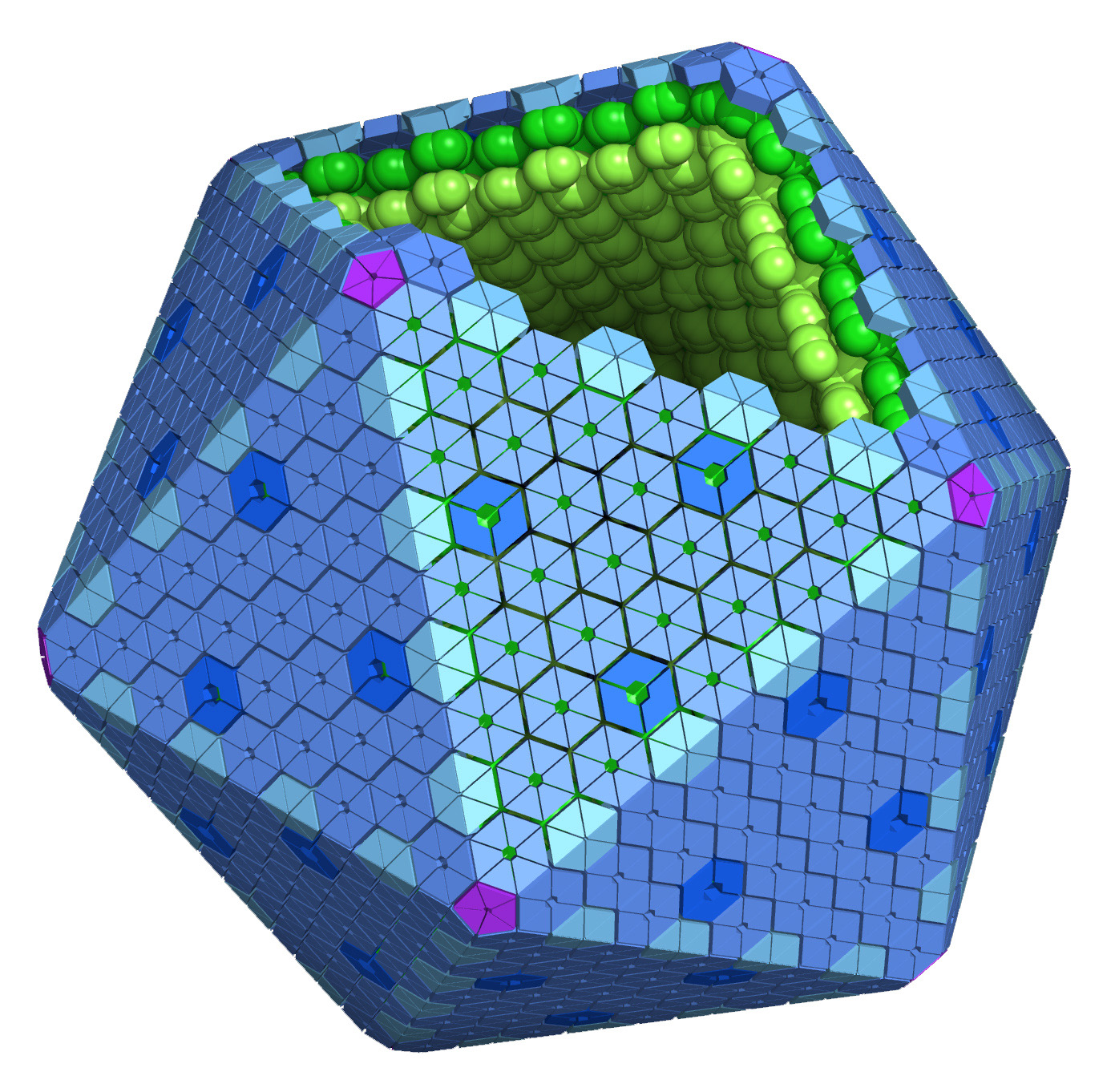
Idealized Microcompartment
Types of MCP Proteins
- BMC-H : Hexameric shell proteins comprised of a single BMC domain
- Permuted BMC: Hexameric shell protein, comprised of a single permuted BMC domain
- BMC-T: Trimeric shell proteins, comprised of a tandem BMC domain
- BMV: Pentameric shell proteins that for the vertices of MCPs
- Enzymes: Internal proteins used to carry out metabolic functions
